Operating On Zero PPM Measurable Nitrates
How Can It Possibly Work? (Or, If It Works, How Can You Argue With It?)
As we mentioned in other articles, in a normal organic aquaponics system, your nitrate level will vary from 1 to 10 ppm (with occasional periods lasting six months or longer in which nitrates are not measurable at all, but the vegetables just keep growing explosively!). The only time we ever see nitrite or nitrate measurements higher than these in an organic aquaponic system is during system startup (in the Startup section of the manual, next). This fact piqued our interest, and helped us develop our current hypothesis, which may explain the difference between nutrient levels in our organic systems and in chemical-based systems (how we refer to systems that use hydroxides for pH adjusters).
In fact, we have operated large (3,600 square feet) commercial aquaponics systems at nitrate levels of 0 (zero!) for six or more months at a time before that level changed (it went up to 3 ppm, whoooo!). The professors, the hydroponicists, and the people who sell overpriced aquaponics systems and greenhouses will say we’re lying; because they’ve never seen a system grow with levels this low (we’ll explain why next).
(Below) A test strip showing nitrate and nitrite levels during a system startup; about 50 ppm nitrates and maybe 0.15 ppm nitrites. That is the ONLY TIME in the life of an organic aquaponic system that you see this kind of nitrate level. Almost all the time the nitrates are under 5 ppm, a lot of the time they are under 2 ppm, and frequently they will measure 1 ppm or zero for months at a time. 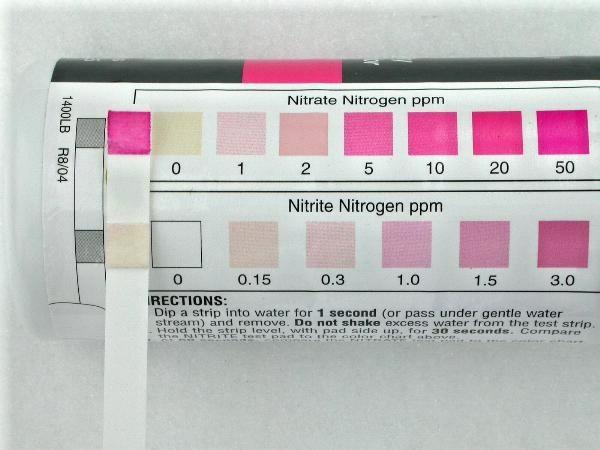
We think the chemical-based university and consultant’s systems show these higher levels of nitrites and nitrates because the repeating applications of caustic chemicals used in them to “balance” pH (calcium hydroxide and potassium hydroxide-lye!) cause repeating die-offs of nitrifying bacteria, which then re-establish themselves in a cycle similar to the nitrite and nitrate spikes that happen once in the life of a normal organic aquaponic system such as ours: during startup.
These repeated cycles of the nitrifying bacteria dying-off and getting re-established cause huge swings in the nitrate levels, which these folks mistake for regular operating levels of nitrates. One of the reasons we figured this out is that in our systems, which were originally built exactly to the university specifications, we were completely unable to “adjust nitrates” the way they explained in their course materials. Our nitrates were all over the place, with no apparent connection to level of fish feeding, net tank cleaning, or pH in the system.
Neither could a student of ours adjust his nitrates using the same methods. At the time, he had also duplicated the operating protocols of the university systems. And the ability to duplicate someone else’s results is the definition of good science. If you can’t, and if other people can’t, by following the same protocols, then the “science” is suspect.
The important thing about seeing these low (and nonexistent!) operating nutrient levels in organic aquaponics is that it throws much of the conventional wisdom out the window. We don’t know exactly what’s happening in our systems. We would love to have a spectrometer, a lab, a year or two, and about $120,000 in salaries and costs for lab workers to get a good handle on what the actual processes are at a microbiological level. What we do understand is that the systems keep growing stuff like crazy even though nitrates are barely measurable (or zero), and they feed us.
And because to be human is to want at least an outline of an explanation of things, here’s what we’ve come up with as a working hypothesis to explain this behavior: the fish are in the system and eating and pooping, so there is decaying organic matter in the water that is passing into the hydroponics troughs.
We always have from 0.25 ppm to 1.0 ppm ammonia in the water, so there is a nutrient source for nitrifying bacteria present. The vegetables are still growing just fine, so they must be getting nutrients from somewhere. We think it starts with the small, suspended bits of decaying organic material (fish poop) that are circulating through the system constantly, and constantly getting turned into ammonia by the system bacteria and chemical processes that do that job.
Next, the bacteria on each root metabolize the ammonia in the system water (that is constantly passing by them) into nitrites and then nitrates, right on the surface of the root itself. The roots then immediately absorb the nutrients. There are no measurable nutrients and very little measurable ammonia in the system water because all this activity is taking place in the few thousandths of an inch of microcosm around the roots of the plants.
When we stick a test strip into the water to measure nitrates, it is miles away (on a bacterial scale) from the bacteria on the root that are making the nitrates. So of course we’re frequently able to measure low or nonexistent nitrate readings in systems that are growing vegetables like crazy! We don’t know of a way to measure the production of nitrates directly on a root itself without an expensive lab and the correct experimental protocols. However, “Zero nitrates” is not a problem in organic aquaponics systems.
Hey guys, my name is wilson brewer. I run multiple very large hobby based systems. One is 650 gal with around 75 plants. I searched for nitrates that hide, and I read your article. My system is running beautifully at 0 nitrates. Tested with a photometer and a API liquid test. I would love to talk to y’all and compare findings in this topic. Thank you