Measuring pH In Your Live Aquaponic System Water
We have started systems with water having a pH as high as 8.3 with no seeming ill effects, so don’t worry if your source water has a high or low pH (also search “aquaponic source water” in the tag cloud to see articles on that subject). The pH starts trending downwards immediately as the fish breathe CO2 into the water and it transmutes into carbonic acid, lowering the pH. When the system pH is down around 6.2 or so, add calcium carbonate as mentioned in the section entitled Nutrient and pH Levels, and continue to monitor pH, adding calcium carbonate as necessary to buffer pH and bring it down.
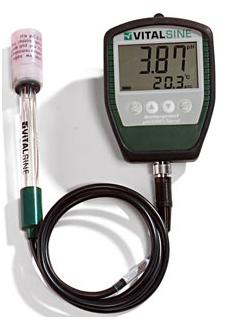
(Below) A VitalSine pH meter, the ONLY accurate way to measure pH! |
A Vital Sine pH meter, about $140 from Aquatic EcoSystems. Allowing the probe to dry out for more than fifteen minutes or so can damage or destroy the probe’s accuracy. When you use it, take all your readings (this will keep the probe wet), then when you are done, store it in the plastic cap filled with storage fluid right away!
Storing the probe end in the plastic cap with storage fluid inside is critical, unless you want to buy new $90 probes on a regular schedule! |
WARNING! Do NOT try to bring down a high pH caused by highly basic water or by having a poorly neutralized concrete tank in your system by using citric acid! Citric acid is an organic herbicide, and will kill your plant’s roots. They all turn black, then the plants all die (courtesy of one of our students whose name we forgot!). We do not yet have a safe and approved method of bringing pH down, just buffering it up with calcium carbonate.
You can use the little pH test strips, or the pH pad on a “multi-strip” to test pH in your system; but we’ve found that those are often quite inaccurate: they will often show as much as 1.5 off what the pH actually was (measured with a pH meter accurate to .01!). In other words, you can’t depend on measurements from these strips! Using an erroneous measurement from a strip or titration test kit may mislead you into doing something damaging to your aquaponics system that you just don’t need to do!
If you have a commercial-scale operation, you should have a good pH meter. The VitalSine pH meter, available from Aquatic EcoSystems for around $140, is the best one we’ve found. Despite the occasional bad review on the web, ours is rock-solid and dependable. In contrast, we got a Milwaukee pH meter for $245 that had great reviews, and it’s never been able to measure pH or even stay calibrated for more than about 10 seconds.
When you buy the pH meter, you also need to buy some pH calibration packets or fluid; this allows you to calibrate the meter so that you know your readings are accurate. You also need to buy a quart of pH electrode storage fluid; that is what you must keep inside the little screw-on cap on the probe. If the probe is out of the cap and dry for even a half hour, it can destroy it. I’ve lost two $90 probes to employees who didn’t put the cap back on after using them.
We never leave the probe in a trough, but put the meter and probe in our test kit basket in a safe dry place every time we finish with it (even though the meter claims to be waterproof). We calibrate it every time we use it by dipping it into a packet of 7.01 Milwaukee calibration/buffer solution I get from Aquatic EcoSystems (30 to a box), then we know the readings are accurate. We rinse the meter probe before putting it in the calibration packet to calibrate it, then carefully close the top of the buffer packet and stick a clothespin on it to hold it closed, storing it upright in a dry location, so we can re-use the calibration packet as many times as possible.
pH in these systems changes slowly, and always towards the direction of acidification (lower pH numbers) so when we see a pH going down into the low 6-range we add a pound or two of calcium carbonate to the system to change the pH upwards towards 7, putting it in right where the water enters the first trough. Calcium carbonate is commonly found in the form of coral sand or crushed oyster shells. We’ve seen the systems be pH-stable for as long as a year and a half between additions.
You may be able to hurt your system by adding too much at a time, but we think this would be difficult, as this carbonate tends to simply precipitate out of the system and lay on the bottoms of troughs or tanks when the pH gets to about 7. It then reabsorbs into the system when the pH goes down over time. When you make additions just do them a bit at a time instead of a barrel.
Warning! Although we haven’t run into it yet, a condition called “nutrient lockout” is caused in soil farming by too high or too low a pH level. If your pH is too high, nutrients such as iron, boron, magnesium, potassium and others become unavailable to the plants because of the interaction at the molecular level. This is called “nutrient lockout”, and they really mean it, because it doesn’t matter how much iron or other supplements you add if your pH is too high. At high enough pH’s, the plants won’t be able to access them, and will continue to exhibit symptoms of nutrient deficiencies although there is plenty of that specific nutrient in your system. Unfortunately, we can’t give you a hard and fast number for what is too high. We DO know right around 7.0 is safe and productive.
Leave a Reply